


 |
 |
 |
| result: | result: | result: |
 |
 |
| result: | result: |
 |
 |
| result: | result: |
|
|
| result: | |
| Analyte Group | Analyte Name | RL | Result | Modifier | Units | MDL | Method | Lab | Date | Time | Lab Comments | QA Flag |
|---|
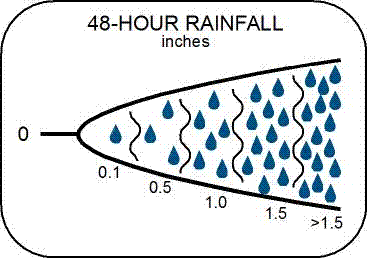
Rainfall - Observed rainfall over the 48 hour period before sampling. Data is also displayed from Weather Underground for the 24 hour period around the sampling date.
On the Watershed Watch field reporting forms, rainfall can be recorded as:
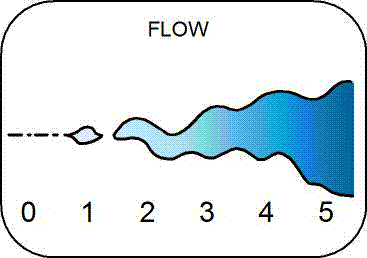
Stream Flow - Flow, also called discharge, refers to the volume of water flowing through a point in the stream per second. Water flowing in streams constitutes land runoff, either over impermeable surfaces, through soils, or from groundwater. Following a rain event, there is often a quick increase in flow due to rainwater reaching the stream, followed by a relatively long decline back to baseflow. The rapid increase in flow can result from rainwater quickly running off of impermeable surfaces or through soils. Alternatively, the rapid increase could be due to rainwater pushing out water that had been stored in wetlands or groundwater. Baseflow is the amount of water that would drain absent any rain inputs, and is usually from groundwater.
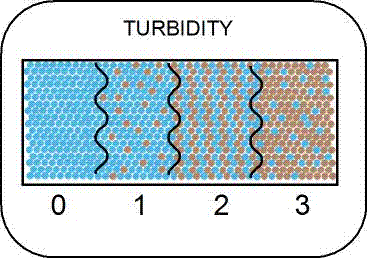
Turbidity - Turbidity is a measure of water clarity and how much the material suspended in the water decreases the passage of light through the water.Suspended materials include soil particles
(clay, silt and sand), algae, plankton, microbes, and other substances.Sources of turbidity include soil erosion, waste discharge, urban runoff, eroding streambanks, large numbers of bottom feeders which
stir up bottom sediments and excessive algal growth (USEPA, https://www.epa.gov/owow/monitoring/volunteer/stream/vms55.html).
Higher turbidity increases water temperatures, because suspended particles absorb more heat.This, in turn, reduces the concentration of dissolved oxygen because warm water holds less dissolved oxygen than cold water.Higher turbidity also reduces the amount of light penetrating the water, which reduces photosynthesis and the production of oxygen.Suspended materials can clog fish gills, reducing resistance to disease in fish, lowering growth rates, and affecting egg and larval development.As the particles settle, they can blanket the stream bottom, especially in slower waters, and smother fish eggs and benthic macroinvertebrates.
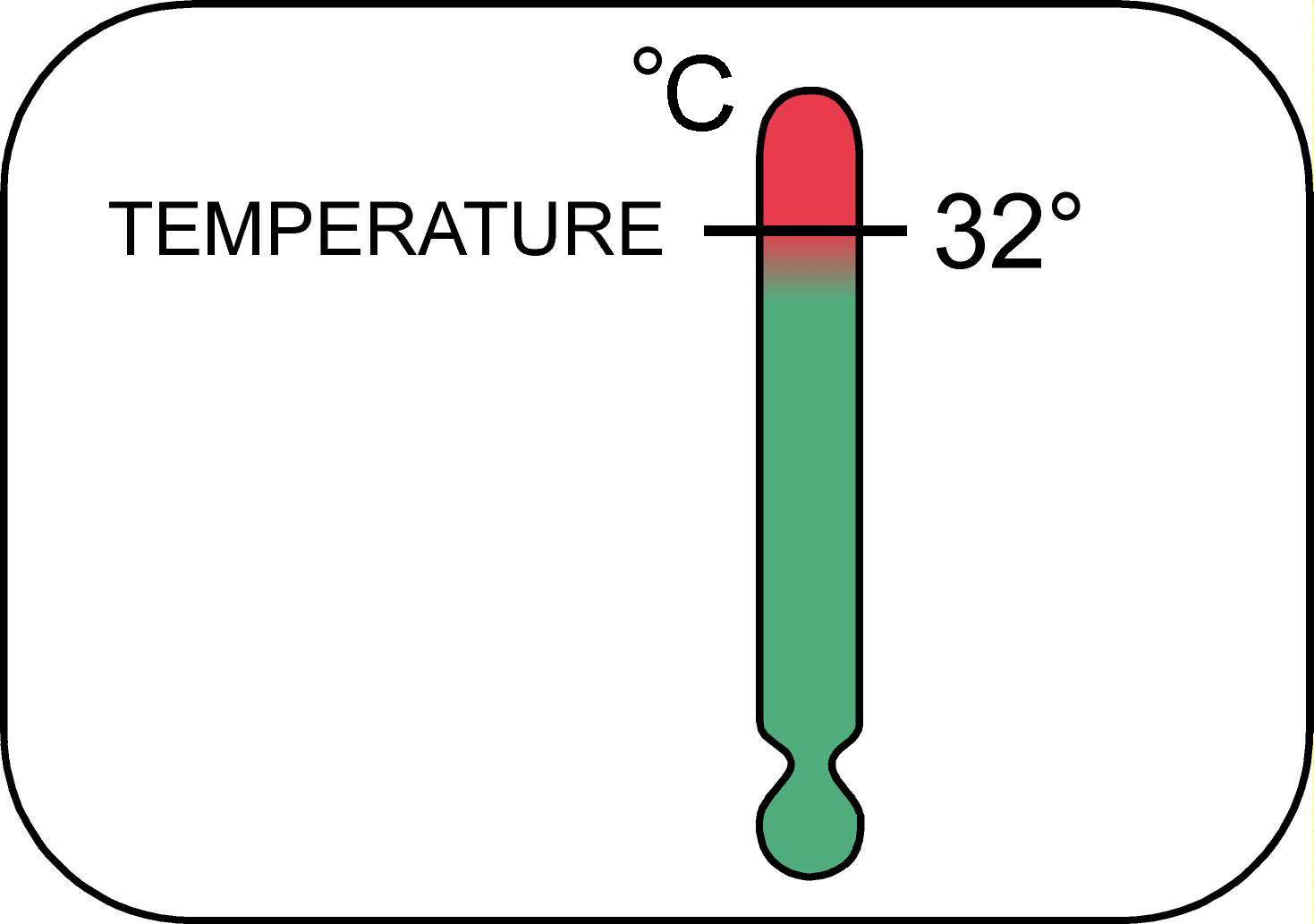
Temperature
Why do we care?
Temperature affects the metabolic processes of aquatic biota and the solubility and toxicity of other parameters. Generally, the solubility of solids increases with increasing temperature, while gases tend to be more soluble in cold water. For example, colder water has a greater capacity to retain dissolved oxygen than warmer water. Temperature is also a factor in determining allowable limits for other parameters, such as ammonia.
Natural occurrence
Temperature varies naturally on a daily and seasonal basis. Natural factors affecting water temperatures in streams include direct sunlight and warm water outflows from shallow ponds or reservoirs. Groundwater - which averages between 55° and 60° F in Kentucky - can influence stream temperature. Mixing of shallow groundwater and surface water commonly occurs in the hyporheic zone, the subsurface area below and adjacent to stream channels where many organisms find refuge during drought and extreme temperatures.
How much matters?
Activities that change water temperatures beyond natural ranges should be avoided, and are prohibited under Clean Water Act rules. Appropriate temperatures are dependent on the type of stream and where it is located. Lowland streams are often categorized as "warmwater" systems, and are different from mountain or spring-fed "coldwater" streams that support organisms with lower temperature and higher oxygen requirements. Temperatures for warmwater streams should not exceed 89° F; coldwater streams should not exceed 68° F.
Higher temperatures can reduce oxygen concentrations and affect growth, reproduction, and metabolic processes in fish and other organisms - sometimes fatally. The table below lists regulatory requirements for seasonal temperature water quality standards.
Human impacts
Removal of shading riparian vegetation and discharges of excessively warm water from industrial treatment facilities, wastewater and power plants, parking lots, roofs, and other areas can affect surface water temperatures. Stormwater infiltration, cooling ponds, and riparian vegetation (e.g., shade trees, shrubs, native grasses) can help to mitigate these effects.
| Month/Date | Period Average | Instantaneous Maximum | (°F) | (°C) | (°F) | (°C) |
|---|---|---|---|---|
| January 1-31 | 45 | 7 | 50 | 10 |
| February 1-29 | 45 | 7 | 50 | 10 |
| March 1-15 | 51 | 11 | 56 | 13 |
| March 16-31 | 54 | 12 | 59 | 15 |
| April 1-15 | 58 | 14 | 64 | 18 |
| April 16-30 | 64 | 18 | 69 | 21 |
| May 1-15 | 68 | 20 | 73 | 23 |
| May 16-31 | 75 | 24 | 80 | 27 |
| June 1-15 | 80 | 27 | 85 | 29 |
| June 16-30 | 83 | 28 | 87 | 31 |
| July 1-31 | 84 | 29 | 89 | 32 |
| August 1-31 | 84 | 29 | 89 | 32 |
| September 1-15 | 84 | 29 | 87 | 31 |
| September 16-30 | 82 | 28 | 86 | 30 |
| October 1-15 | 77 | 25 | 82 | 28 |
| October 16-31 | 72 | 22 | 77 | 25 |
| November 1-30 | 67 | 19 | 72 | 22 |
| December 1-31 | 52 | 11 | 57 | 14 |
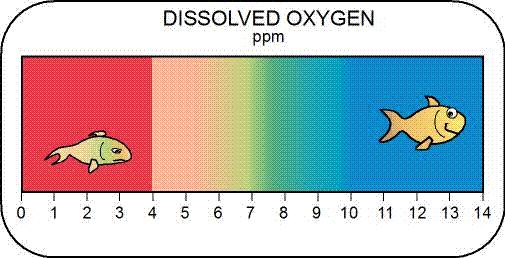
Dissolved Oxygen
Why do we care?
Oxygen is one of the most important constituents in aquatic systems, because it supports metabolic processes of aerobic organisms (e.g., fish, insect larva) and controls many inorganic and organic chemical reactions.
Natural occurrence
Oxygen is constantly being exchanged between surface waters and the atmosphere, through diffusion, aeration, (vertical mixing of water, as in riffles), and via oxygen-containing inorganic and organic compounds. The maximum possible concentration of dissolved oxygen (DO) in water is controlled by atmospheric pressure and water temperature. Oxygen saturation in water is proportional to atmospheric pressure and inversely proportional to water temperature, so the cooler the water the greater the DO concentration.
Biologic processes add and remove oxygen from a waterbody. In a nutrient-rich water body, the DO can be quite high in the surface water during the day due to photosynthesis from algae and aquatic plants, and low at night due microorganism respiration (biologically mediated oxidation). DO can also be chemically removed - or bound up - by oxidation of other constituents in the water, such as iron.
Because of daily temperature cycles and because photosynthesis and respiration is controlled by sunlight, the DO concentration in water tends to undergo diurnal cycles: DO is higher during periods of sunlight, and lower at night.
In aquatic systems, the physical, chemical and biologic processes interact in complex ways. For example, DO tends to be depleted in deeper waters because photosynthesis is reduced owing to poor light penetration and the fact that dead algae (phytoplankton) sinks, and is decomposed by aerobic bacteria and other microorganisms.
How much matters?
DO values less than 5 mg/L are problematic over time for aquatic organisms, resulting in increased susceptibility to environmental stresses, reduced growth rates, mortality and an alteration in the distribution of aquatic life. Levels that remain below 1-2 mg/L for a few hours can result in severe fish kill. A grab sample reading should be compared with the instantaneous minimum (acute) criteria, which is 4 mg/L. Mountain or spring-fed streams that are designated as cold-water aquatic habitat (i.e., for trout, etc.) require higher DO.
Human impacts on concentrations
Water temperature increases caused by removal of riparian shade trees, power plant releases, or urban stormwater decreases instream DO. Increased nutrient levels can cause excessive algal growth, resulting in decreased DO as algae dies and is decomposed by aerobic biota.
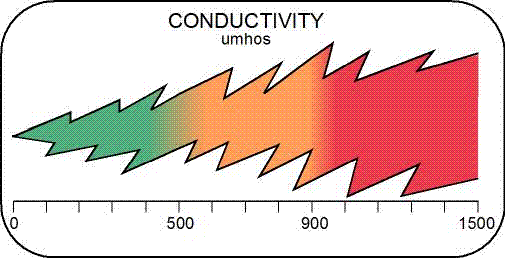
Field Conductivity - Conductivity is a measurement of the ability of an aqueous solution to carry an electrical current. Conductivity measurements are used to determine levels of total inorganic dissolved solid ions, such as nutrients, metals, or other compounds.Indirect effects of high conductivity levels are primarily the elimination of plants needed for food or habitat and the decline of sensitive aquatic species, such as mayflies and fish.
The EPA's newly established conductivity criterion for streams in Central Appalachia is 500 micromohs/cm.In central Appalachia, the conductivity of headwater streams is naturally between 100 micromhos/cm and 200 micromhos/cm This is important because the plants, insects and animals in local streams have adapted to living in this level of conductivity.Recent studies conducted by the EPA show that when the conductivity in central Appalachian streams rises to about 300 micromhos/cm, the plants, insects and animals begin to be affected. When the conductivity of these streams goes above 500 micromhos/cm, the plants, insects and animals are drastically affected. And when the conductivity measures above 1,000 micromhos/cm, everything in the stream is effectively dead. [NOTE: KDOW sampling has shown that some pollutant-tolerant aquatic life is present at conductivity levels greater than 1,000.micromhos/cm]In other regions of the country the natural conductivity may be higher or lower than in central Appalachia, and the plants, insects and animals there will have adapted over thousands of years to live within those natural conductivity levels.
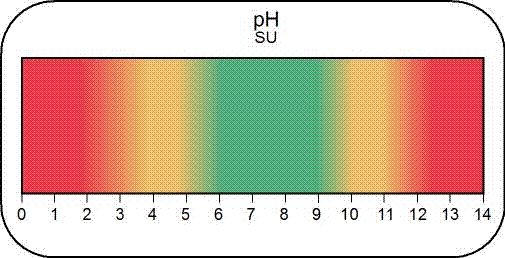
pH
Why do we care?
The pH of water is a measurement showing how acidic or alkaline the water is, which affects a waterbody's ability to support aquatic life, as well as the water's usefulness for domestic or industrial purposes. pH is also measured as an indicator of increasing pollution or other environmental impacts.
The pH of water determines the solubility (amount that can be dissolved in the water) and biological availability (amount that can be utilized by aquatic life) of chemical constituents such as nutrients (phosphorus, nitrogen, and carbon) and heavy metals (lead, copper, cadmium, etc.). For example, in addition to affecting how much and what form of phosphorus is most abundant in the water, pH also determines whether aquatic life can use it. In the case of heavy metals, the degree to which they are soluble determines their toxicity. Metals tend to be more toxic at lower pH, because they are more soluble. (Source: A Citizen's Guide to Understanding and Monitoring Lakes and Streams)
Natural occurrence
A waterbody's pH level can be affected by natural conditions, such as rainfall or precipitation, bedrock composition, surrounding vegetation, and other factors. The average pH of rainfall is 5.6, indicating its slight acidity due to carbon dioxide (CO2) in the atmosphere forming carbonic acid as CO2 interacts with water. The pH of water can also be lowered from the contribution of tannic acids from decaying plant matter from species that are high in tannins, such as pine, cedar, red oak or willows.
Alternatively, bedrock composed of carbonate rock, such as limestone, can increase the alkalinity of water that flows over or through it. Waters in limestone-rich areas, therefore, have a "buffering capacity" that can help neutralize acidic inputs, such as acid rain or mining runoff.
How much matters?
The pH scale ranges from 1 (very acidic) to 14 (very alkaline). The pH of pure water is 7, and the normal range for surface water is between 6 and 9. A pH of 6 or lower signifies acidic conditions in which toxic heavy metals are more soluble and, therefore, more available for uptake by aquatic life. At pH values of 9 or greater, conditions become alkaline and toxic levels of ammonia (NH3) may become problematic for aquatic life.
Human impacts on concentrations
The pH of waterways can be altered by chemical inputs from residential or commercial sources. Many industrial processes require water that has a very specific pH level. Once the water is used, it may be returned to a nearby waterway, affecting its pH level. Air pollutants which combine with precipitation to form acid rain also affect pH. Nitrogen oxides (NOx) and sulfur dioxide (SO2) from automobile and coal-fired plant emissions interact with water vapor to form acid rain. Another human activity affecting pH is coal mining, which may produce drainage of sulfuric acid, as iron sulfides in the coal seams combine with water. Coal mining runoff can drastically reduce the pH of nearby streams.
Sources
E. coli - The bacteria, E. coli, is commonly found in the intestines of healthy humans and animals and produces the K and B C complex vitamins that are then absorbed for nutritional benefit.The presence of E. coli in water indicates fecal contamination and the potential for waterborne disease.EPA recommends E. coli as the best indicator of health risk from water contact in recreational waters.Kentucky has transitioned from a fecal coliform standard to an E. coli standard.[Recently, Akasapu and Ormsbee (2011) developed a mathematical approximation between fecal coliform values (FC) and E. Coli values for samples in the Kentucky River Basin which can be used to relate past fecal coliform values to equivalent E. coli values.The relationship is:E.coli= 1.435*FC0.8093 ]
The state criteria for E. coli are based on the designated use of the particular stream and may be summarized as follows: Primary Contact Recreation (swimming from May 1 thru Oct 31): E. coli shall not exceed 130 colonies per 100 ml as a monthly geometric mean based on not less than 5 samples per month; nor exceed 240 colonies per 100 ml in 20 percent or more of all samples taken during the month [Note: As a result of the sampling frequency requirement with the first criteria, the state of Kentucky uses the 240 colonies per 100-ml criteria for classifying streams in the 305(b) report].
There is no description yet for this analyte.
There is no description yet for this analyte.
2,4-Dichlorophenoxyacetic acid (2,4-D) - 2,4-D is a common systemic herbicide used in the control of broadleaf weeds. It is the most widely used herbicide in the world, and the third most commonly used in North America.According to the US EPA's website, the short-term health effects of exposure to high levels of 2,4-D in drinking water can include nervous system damage.Long-term exposure to 2,4-D can potentially cause damage to the nervous system, kidney and liver.
There is no description yet for this analyte.
There is no description yet for this analyte.
Triazine - Triazine (or Atrazine) is a selective triazine herbicide used to control broadleaf and grassy weeds in corn and other crops, and in conifer reforestation plantings.It is also used as a nonselective herbicide on non-cropped industrial lands and on fallow lands. Atrazine is moderately soluble in water.Atrazine is highly persistent in soil.Chemical hydrolysis followed by microbial breakdown accounts for most of its degradation in soil.Although hydrolysis is rapid in acidic or basic soil environments, it is slower at neutral pHs.
The USEPA's Drinking Water Maximum Contaminant Level for atrazine is 3 micrograms/L.
The procedures described in the "Guidelines for Deriving Numerical National Water Quality Criteria for the Protection of Aquatic Organisms and Their Uses" indicate that, except possibly where a locally important species is very sensitive, freshwater aquatic life and their uses should not be affected unacceptably if the one-hour average concentration does not exceed 350 ug/L more than once every three years on the average (acute criterion). If the four-day average concentration of atrazine does not exceed 12 ug/L more than once every three years on the average (chronic criterion).
There is no description yet for this analyte.
Nitrate (NO3)
Why do we care?
The most important plant nutrients, in terms of water quality, are nitrogen and phosphorus. Nitrate is the product of aerobic transformation of ammonia, and is the most common form of nitrogen used by aquatic plants. In general, increasing nutrient concentrations increases the potential for accelerated growth of aquatic plants, including algae. Nuisance plant growth can create imbalances in the aquatic community, as well as cause aesthetic and access issues. High densities of phytoplankton (algae) can cause wide fluctuations in pH and dissolved oxygen.
Natural occurrence
Nitrogen is one of the most widely distributed elements in nature and is present virtually everywhere on the earth's crust in one or more of its many chemical forms. Nitrate (NO3), a mobile form of nitrogen, is commonly found in ground and surface waters throughout the country. Nitrate is generally the dominant form of nitrogen where total nitrogen levels are elevated. Nitrate and other forms of nitrogen in water can be from natural sources, but when concentrations are elevated, the sources are typically associated with human activities (Dubrovski et al., 2010).
How much matters?
The state drinking water supply standard for nitrate-nitrogen, which is a measurement of the nitrogen portion of the nitrate (NO3) molecule, is 10 mg/L. Nitrates in drinking water can react directly with hemoglobin in the blood of humans and other warm-blooded animals to produce methemoglobin, which destroys the ability of red blood cells to transport oxygen. This condition is especially serious in babies under three months of age and causes a condition known as methemoglobinemia, or "blue baby" disease.
In order to monitor nutrient effects on aquatic life, the Kentucky River Watershed Watch organization is using a proposed standard of 3 mg/L for total nitrogen, because this level has been demonstrated to produce nutrient-rich conditions supporting algal blooms, along with other aquatic habitat threats.
Human impacts on concentrations
Oxygen demanding materials and plant nutrients are among the most common substances discharged to the environment by human activities, through wastewater facilities (sewage treatment and industrial water treatment) and by agricultural, residential, and stormwater runoff. Agricultural and residential fertilizers and lawn treatment spray are potential sources of nitrates in stormwater runoff water. Failing septic systems can also release excess nitrogen in sewage runoff, as can sewer system overflows and inadequate sewage treatment plants.
Sources
Nitrogen in Waters: Forms and Concerns, Dave Wall, Minnesota Pollution Control Agency
The quality of our Nation's waters - Nutrients in the Nation's streams and groundwater, 1992 - 2004: U.S. Geological Survey Circular 1350, Dubrovski et al.
There is no description yet for this analyte.
There is no description yet for this analyte.
There is no description yet for this analyte.
Total Nitrogen (N)
Why do we care?
In general, increasing nutrient concentrations (mainly nitrogen and phosphorus) increases the potential for accelerated growth of aquatic plants, including algae. Nuisance plant growth can create imbalances in the aquatic community, as well as cause aesthetic and access issues. High densities of phytoplankton (algae) can cause wide fluctuations in pH and dissolved oxygen.
Natural occurrence
Nitrogen is one of the most widely distributed elements in nature and is present virtually everywhere on the earth's crust in one or more of its many chemical forms. Nitrogen enters water in numerous inorganic and organic forms. Since nitrogen can transform from one chemical composition to another, it is often considered in its totality as total nitrogen (TN).
The primary inorganic forms of nitrogen are ammonia, ammonium, nitrate, and nitrite. Organic-nitrogen (organic-N) is found in proteins, amino acids, urea, living or dead organisms (i.e., algae and bacteria) and decaying plant material. Organic-N is usually determined from the laboratory method called total Kjeldahl nitrogen (TKN), which measures a combination of organic N and ammonia+ ammonium. (Nitrogen in Waters: Forms and Concerns, https://www.pca.state.mn.us/sites/default/files/wq-s6-26a2.pdf, Dave Wall, Minnesota Pollution Control Agency)
How much matters?
Kentucky currently has no official numerical standard or criteria for total nitrogen in state waterways, but is working toward developing these standards. In order to monitor nutrient effects on aquatic life, Kentucky River Watershed Watch is using a proposed standard of 3 mg/L for total nitrogen, because this level has been demonstrated to produce nutrient-rich conditions supporting algal blooms, along with other aquatic habitat threats.
Human impacts on concentrations
In most surface waters, the dominant forms of nitrogen are nitrate and organic-N. Where streams originate in areas of agricultural production, the nitrate form is usually substantially higher than organic nitrogen. Because nitrate is very low in forested and grassland areas, organic nitrogen is typically higher than nitrate in landscapes dominated by these more natural conditions. Ammonia and ammonium forms of nitrogen are usually only elevated near sources of human or animal waste discharges. (Nitrogen in Waters: Forms and Concerns, Wall, MPCA)
Sources
Nitrogen in Waters: Forms and Concerns, Dave Wall, Minnesota Pollution Control Agency
The quality of our Nation's waters - Nutrients in the Nation's streams and groundwater, 1992 - 2004: U.S. Geological Survey Circular 1350, Dubrovski et al.
There is no description yet for this analyte.
Total Phosphorus - Total phosphorus is commonly measured to determine phosphorus concentrations in surface waters.This measurement ncludes all of the various forms of phosphorus (organic, inorganic, dissolved, and particulate) present in a sample. Phosphorus is one of the key elements necessary for growth of plants and animals. Phosphates are made up of phosphorus and exist in three forms: orthophosphate, metaphosphate (or polyphosphate) and organically bound phosphate. Each compound contains phosphorous in a different chemical formula. Ortho forms are produced by natural processes and are found in sewage. Poly forms are used for treating boiler waters and in detergents. In water, they change into the ortho form.Organic phosphates are important in nature.Their occurrence may result from the breakdown of organic pesticides that contain phosphates.They may exist in solution, as particles, loose fragments, or in the bodies of aquatic organisms.
In addition to man-made sources, some phosphorus loadings may occur naturally from the watershed soils and underlying geology.Due to background levels of total phosphorus in the Kentucky River Basin as high as 0.25 mg/L, those sites with average total phosphorus concentrations of 0.3 mg/L can be noted as potentially problematic.The informal total phosphorus standard of 0.3 mg/L has been adopted by the KRWW Scientific Advisory Committee as an appropriate level of concern for water quality sampling conducted in the Kentucky River Basin.This value has also been recommended for use by the Kentucky Division of Water.
Total Recoverable Phosphorus (P)
Why do we care?
The most important plant nutrients, in terms of water quality, are phosphorus and nitrogen. In general, increasing nutrient concentrations increases the potential for accelerated growth of aquatic plants, including algae. Nuisance plant growth can create imbalances in the aquatic community, as well as cause aesthetic and access issues. High densities of phytoplankton (algae) can cause wide fluctuations in pH and dissolved oxygen.
Total phosphorus is commonly measured to determine phosphorus concentrations in surface waters, and includes all of the various forms of phosphorus (organic, inorganic, dissolved, and particulate) present in a sample. Phosphorus is one of the key elements necessary for growth of plants and animals.
Natural occurrence
Some phosphorus loadings may occur naturally from the watershed soils and underlying geology. Organic phosphates are important in nature. They may exist in solution, as particles, loose fragments, or in the bodies of aquatic organisms.
In Central Kentucky, the limestone geology frequently contributes significant phosphorus to waters running through and over the bedrock. It is important to consider this factor when assessing total phosphorus concentrations in relation to water quality and factor in an allowance for naturally occurring phosphorus.
How much matters?
Kentucky currently has no official numerical standards or criteria for phosphorus in state waterways, but is working toward developing these standards. Due to background total phosphorus levels in the Kentucky River Basin of as high as 0.25 mg/L, those sites with average total phosphorus concentrations of 0.3 mg/L can be noted as potentially problematic to aquatic life support. The informal benchmark of 0.3 mg/L has been adopted by the Kentucky River Watershed Watch Scientific Advisory Committee as an appropriate level of concern for water quality sampling conducted in the Kentucky River Basin. It is believed that total phosphorus levels above this level can contribute to harmful algal blooms in the waterbody.
Human impacts on concentrations
Human sources of phosphorus in waterways include sewage, boiler waters and detergents, and the breakdown of commercial pesticides.
Sources
A Summary of the Kentucky River Watershed Watch 2014 Water Sampling Results, Ormsbee and McAlister, Kentucky Water Resources Research Institute
Sulfur - Sulfur is another essential plant nutrient. Aquatic organisms utilize sulfur, and reduced concentrations have a detrimental effect on algal growth. The most common form of sulfur in well-oxygenated waters is sulfate. Sulfates (SO4--) can be naturally occurring or the result of municipal or industrial discharges. When naturally occurring, they are often the result of the breakdown of leaves that fall into a stream, of water passing through rock or soil containing gypsum and other common minerals, or of atmospheric deposition. Point sources include sewage treatment plants and industrial discharges such as tanneries, pulp mills, and textile mills. Runoff from coal mining operations and fertilized agricultural lands also contributes sulfates to water bodies.
A sulfur cycle exists, which includes atmospheric sulfur dioxide (SO2), sulfate ions (SO22-) and sulfides (S-). Sulfides, especially hydrogen sulfide (H2S), are quite soluble in water and are toxic to both humans and fish. They are produced under conditions where there is a lack of oxygen (anaerobic). Because of their foul "rotten egg" smell they are avoided by both fish and humans. Sulfides formed as a result of acid mine runoff from coal or other mineral extraction and from industrial sources may be oxidized to form sulfates, which are less toxic.
When sulfate is less than 0.5 mg/L, algal growth will not occur. On the other hand, sulfate salts can be major contaminants in natural waters.The state water quality standard for sulfate in drinking water supplies is 250 mg/L.
Total Kjeldahl Nitrogen (N)
Why do we care?
In general, increasing nutrient concentrations (mainly nitrogen and phosphorus) increases the potential for accelerated growth of aquatic plants, including algae. Nuisance plant growth can create imbalances in the aquatic community, as well as cause aesthetic and access issues. High densities of phytoplankton (algae) can cause wide fluctuations in pH and dissolved oxygen.
Natural occurrence
Nitrogen is one of the most widely distributed elements in nature and is present virtually everywhere on the earth's crust in one or more of its many chemical forms. Nitrogen enters water in numerous inorganic and organic forms. Since nitrogen can transform from one chemical composition to another, it is often considered in its totality as total nitrogen (TN).
The primary inorganic forms of nitrogen are ammonia, ammonium, nitrate, and nitrite. Organic-nitrogen (organic-N) is found in proteins, amino acids, urea, living or dead organisms (i.e., algae and bacteria) and decaying plant material. Organic-N is usually determined from the laboratory method called total Kjeldahl nitrogen (TKN), which measures a combination of organic N and ammonia+ ammonium. (Nitrogen in Waters: Forms and Concerns, https://www.pca.state.mn.us/sites/default/files/wq-s6-26a2.pdf, Dave Wall, Minnesota Pollution Control Agency)
How much matters?
Kentucky currently has no official numerical standard or criteria for total nitrogen in state waterways, but is working toward developing these standards. In order to monitor nutrient effects on aquatic life, Kentucky River Watershed Watch is using a proposed standard of 3 mg/L for total nitrogen, because this level has been demonstrated to produce nutrient-rich conditions supporting algal blooms, along with other aquatic habitat threats.
Human impacts on concentrations
In most surface waters, the dominant forms of nitrogen are nitrate and organic-N. Where streams originate in areas of agricultural production, the nitrate form is usually substantially higher than organic nitrogen. Because nitrate is very low in forested and grassland areas, organic nitrogen is typically higher than nitrate in landscapes dominated by these more natural conditions. Ammonia and ammonium forms of nitrogen are usually only elevated near sources of human or animal waste discharges. (Nitrogen in Waters: Forms and Concerns, Wall, MPCA)
Sources
Nitrogen in Waters: Forms and Concerns, Dave Wall, Minnesota Pollution Control Agency
The quality of our Nation's waters - Nutrients in the Nation's streams and groundwater, 1992 - 2004: U.S. Geological Survey Circular 1350, Dubrovski et al.
There is no description yet for this analyte.
Antimony is a USEPA priority pollutant that can be toxic to plants and animals. In addition to the natural occurrence of antimony in bedrock and streambed sediments in the Knobs Region of the Kentucky River Basin, antimony salts are used in the fireworks, rubber, textile, ceramic, glass, and paint industries.The proposed maximum contaminant level (MCL) in finished drinking water for antimony ranges from 5 to 10 micrograms per liter.
Arsenic occurs naturally in rocks and soil, water, air and plants and animals.It can be further released into the environment through natural activities, such as volcanic action, erosion of rocks, and forest fires, or through human actions.Approximately 90 percent of industrial arsenic in the U.S. is currently used as a wood preservative, but arsenic is also used in paints, dyes, metals, drugs, soaps and semi-conductors.High arsenic levels can also come from certain fertilizers and animal feeding operations.Industry practices, such as copper smelting, mining and coal burning also contribute to arsenic in our environment.Arsenic levels tend to be higher in ground water than in surface water (lakes and rivers).Levels also tend to be higher in the western United States.
Barium is a yellowish-white alkaline earth metal. It combines with water to produce barium hydroxide and is found in nature as barites (BaSO4), witherite (BaCO3), and other ores. Barium and its salts are often used in metallurgical industries for special alloys, in paints, and concrete.Because of the insolubility of most of its compounds, it is not considered to be an ecological threat.
Beryllium is an uncommon alkaline-earth element that is recognized as a USEPA priority pollutant and potential carcinogen.The USEPA has proposed a MCL of 1.0 micrograms per liter for beryllium, and Kentucky has adopted the USEPA lowest-observed effect levels (LOEL) for protection of aquatic life, which are 130 micrograms/liter (1.3 mg/L) and 5.3 micrograms/liter (0.053 mg/L) for acute and chronic toxicity, respectively. In addition, Kentucky water-quality criteria establish a beryllium criterion of 0.117 micrograms per liter for the protection of human health from the consumption of fish tissue. The criterion is based upon an acceptable risk level of no more than one additional cancer case in a population of 1 million people.
There is no description yet for this analyte.
Cadmium is a non-essential element and it diminishes plant growth. It is considered a potential carcinogen. It also has been shown to cause toxic effects to the kidneys, bone defects, high blood pressure, and reproductive effects.Cadmium is widely distributed in the environment at low concentrations. It can be found in fairly high concentrations in sewage sludge. Primary industrial uses for cadmium are plating, battery manufacture, pigments, and plastics.
There is no description yet for this analyte.
Chromium is ubiquitous in the environment, occurring naturally in the air, water, rocks and soil. It is used in stainless steel, electroplating of chrome, dyes, leather tanning and wood preservatives. It occurs in several forms, or oxidation states. The two most common are chromium VI and chromium III. The form depends on pH. Natural sources of water contain very low concentrations of chromium. It is a micronutrient (or essential trace element). High doses of chromium VI have been associated with birth defects and cancer; however, chromium III is not associated with these effects. Plants and animals do not bioaccumulate chromium; therefore, the potential impact of high chromium levels in the environment is acute toxicity to plants and animals. In animals and humans this toxicity may be expressed as skin lesions or rashes and kidney and liver damage.
There is no description yet for this analyte.
Copper is a USEPA priority pollutant that is a micronutrient for the growth of plants and animals, but even small concentrations of copper in surface water can be toxic to aquatic life.Copper sulfate is frequently used to control nuisance growths of algae in water supply reservoirs.The toxicity of copper is a function of the total hardness of the water, because copper ions are complexed by anions that contribute to water hardness.Although detectable concentrations of copper in water are not known to have an adverse effect on humans, the MCL for copper has been established at 1,000 micrograms/liter, which corresponds with the taste threshold concentration for this element.
There is no description yet for this analyte.
Iron is the fourth most abundant element, by weight, in the earth's crust. Natural waters contain variable amounts of iron depending on the geological area and other chemical components of the waterway. Iron in groundwater is normally present in the ferrous or bivalent form (Fe2+), which is soluble. It is easily oxidized to ferric iron (Fe3+) or insoluble iron upon exposure to air. This precipitate is orange-colored and often turns streams orange.Iron is a trace element required by both plants and animals. It is a vital part of the oxygen transport mechanism in the blood (hemoglobin) of all vertebrate and some invertebrate animals.Ferrous Fe2+ and ferric Fe3+ irons are the primary forms of concern in the aquatic environment.Other forms may be in either organic or inorganic wastewater streams. The ferrous form can persist in water void of dissolved oxygen and usually originates from groundwater or mines that are pumped or drained. Iron in domestic water supply systems stains laundry and porcelain. It appears to be more of a nuisance than a potential health hazard. Taste thresholds of iron in water are 0.1 mg/L for ferrous iron and 0.2 mg/L for ferric iron, giving a bitter taste or an astringent taste. Water to be used in industrial processes should contain less than 0.2 mg/L iron. Black or brown swamp waters may contain iron concentrations of several mg/L in the presence or absence of dissolved oxygen, but this iron form has little effect on aquatic life.
Lead is primarily found in nature as the mineral galena (lead sulfide). It also occurs as carbonate, as sulfate and in several other forms. The solubility of these minerals and also of lead oxides and other inorganic salts is low. Major modern day uses of lead are for batteries, pigments, and other metal products. In the past, lead was used as an additive in gasoline and became dispersed throughout the environment in the air, soils, and waters as a result of automobile exhaust emissions. For years, this was the primary source of lead in the environment. However, since the replacement of leaded gasoline with unleaded gasoline in the mid-1980's, lead from that source has virtually disappeared. Mining, smelting, and other industrial emissions and combustion sources and solid waste incinerators are now the primary sources of lead. Another source of lead is paint chips and dust from buildings built before 1978 and from bridges and other metal structures.
There is no description yet for this analyte.
There is no description yet for this analyte.
There is no description yet for this analyte.
Nickel is a USEPA priority pollutant that can adversely affect humans and aquatic organisms.Nickel is an important industrial metal that is used extensively in stainless steel. Substantial amounts of nickel can be contributed to the environment by waste disposal and atmospheric emissions. Nickel ions are toxic, particularly to plant life, and can exhibit synergism when present with other metallic ions.
There is no description yet for this analyte.
There is no description yet for this analyte.
Selenium is a nonmetallic trace element that is listed as a primary pollutant by the USEPA.Selenium is an essential micronutrient for plants and animals, but can be toxic in excessive amounts. Selenium is a relatively rare element, and concentrations of selenium in natural waters seldom exceed 1.0 microgram/liter. Sources of selenium in the Kentucky River Basin include sedimentary rocks and fly ash from coal-fired power plants that operate in Kentucky.
There is no description yet for this analyte.
Silver is a USEPA priority pollutant that is extensively used for photography and various industrial and commercial purposes. Although average concentrations of silver in natural waters are small (0.3 micrograms/liter), elevated silver concentrations can be acutely or chronically toxic to aquatic organisms, and sublethal amounts can bioaccumulate in fish and invertebrate organisms.
There is no description yet for this analyte.
There is no description yet for this analyte.
Sulfur - Sulfur is another essential plant nutrient. Aquatic organisms utilize sulfur, and reduced concentrations have a detrimental effect on algal growth. The most common form of sulfur in well-oxygenated waters is sulfate. Sulfates (SO4--) can be naturally occurring or the result of municipal or industrial discharges. When naturally occurring, they are often the result of the breakdown of leaves that fall into a stream, of water passing through rock or soil containing gypsum and other common minerals, or of atmospheric deposition. Point sources include sewage treatment plants and industrial discharges such as tanneries, pulp mills, and textile mills. Runoff from coal mining operations and fertilized agricultural lands also contributes sulfates to water bodies.
A sulfur cycle exists, which includes atmospheric sulfur dioxide (SO2), sulfate ions (SO22-) and sulfides (S-). Sulfides, especially hydrogen sulfide (H2S), are quite soluble in water and are toxic to both humans and fish. They are produced under conditions where there is a lack of oxygen (anaerobic). Because of their foul "rotten egg" smell they are avoided by both fish and humans. Sulfides formed as a result of acid mine runoff from coal or other mineral extraction and from industrial sources may be oxidized to form sulfates, which are less toxic.
When sulfate is less than 0.5 mg/L, algal growth will not occur. On the other hand, sulfate salts can be major contaminants in natural waters.The state water quality standard for sulfate in drinking water supplies is 250 mg/L.
Thallium is a USEPA priority pollutant that can be toxic to humans and aquatic life. Thallium salts are used as poison for rats and other rodents, as well as in dyes, pigments in fireworks, and optical glass .
There is no description yet for this analyte.
There is no description yet for this analyte.
Zinc is found naturally in many rock-forming minerals. Because of its use in the vulcanization of rubber, it is generally found at higher levels near highways. It also may be present in industrial discharges. It is used to galvanize steel, and is found in batteries, plastics, wood preservatives, antiseptics, and in rat and mouse poison (zinc phosphide).Zinc is an essential element in the diet. It is not considered very toxic to humans or other organisms.
Alkalinity - Alkalinity refers to the degree to which the water sample is basic, or has a pH greater than 7, and affects the capability of water to neutralize acid.In most natural water bodies in Kentucky the buffering system is carbonate-bicarbonate.Alkalinity is important for fish and aquatic life because it protects or buffers against rapid pH changes.Higher alkalinity levels in surface waters will buffer acid rain and other acid wastes and prevent pH changes that are harmful to aquatic life.Kentucky's water quality criteria state that for protection of aquatic life, the buffering capacity should be at least 20 mg/L.If alkalinity is naturally low, (less than 20 mg/L) there can be no greater than a 25% reduction in alkalinity.
Bromide (Br-, an ion)
Why do we care?
A raised concentration of bromides can indicate human-based sources. Bromides can interact with drinking water disinfection chemicals and naturally occurring organic matter in drinking-water, and can contribute to elevated brominated and mixed chloro-bromo byproducts, such as trihalomethanes (THM's) and disinfection by-products that are regulated by US EPA.
Natural occurrence
Bromide is commonly found in nature along with sodium chloride, owing to their similar physical and chemical properties, but in smaller quantities.1 Br- concentrations in natural ecosystems are usually very small.2 Concentrations in fresh water range from trace amounts to about 0.5 mg/l.3
How much matters?
There is no regulatory value for bromide in waterways or drinking water. Bromide ion has a low degree of toxicity; thus, bromide is not of toxicological concern in nutrition. It rarely occurs in concentrations that would pose health risk.4 However, its application on a larger scale requires a careful evaluation of the concentration to be expected in ground and surface water. A quality criterion for groundwater of 1 mg Br- L-1 has been established in the literature.5
Human impacts on concentrations
Bromides can be elevated as a result of brine discharges related to oil and gas exploration and drilling. Other sources are leaded fuel and the use of fertilizers and pesticides in agriculture. Bromides can also be elevated from the production of dyes, pharmaceuticals and other products (Kittel, 1983).6
Chlorides - Chlorides are salts resulting from the combination of the gas chlorine with a metal.Fish and aquatic communities cannot survive in waters with high levels of chlorides.The state of Kentucky requires that chloride levels be less than 250 mg/L in domestic water supplies. Criteria for protection of aquatic life require levels of less than 600 mg/L for chronic (long-term) exposure and 1200 mg/L for short-term exposure.
Conductivity - Conductivity is a measurement of the ability of an aqueous solution to carry an electrical current. Conductivity measurements are used to determine levels of total inorganic dissolved solid ions, such as nutrients, metals, or other compounds.Indirect effects of high conductivity levels are primarily the elimination of plants needed for food or habitat and the decline of sensitive aquatic species, such as mayflies and fish.
The EPA's newly established conductivity criterion for streams in Central Appalachia is 500 micromohs/cm.In central Appalachia, the conductivity of headwater streams is naturally between 100 micromhos/cm and 200 micromhos/cm This is important because the plants, insects and animals in local streams have adapted to living in this level of conductivity.Recent studies conducted by the EPA show that when the conductivity in central Appalachian streams rises to about 300 micromhos/cm, the plants, insects and animals begin to be affected. When the conductivity of these streams goes above 500 micromhos/cm, the plants, insects and animals are drastically affected. And when the conductivity measures above 1,000 micromhos/cm, everything in the stream is effectively dead. [NOTE: KDOW sampling has shown that some pollutant-tolerant aquatic life is present at conductivity levels greater than 1,000.micromhos/cm]In other regions of the country the natural conductivity may be higher or lower than in central Appalachia, and the plants, insects and animals there will have adapted over thousands of years to live within those natural conductivity levels.
There is no description yet for this analyte.
Total Suspended Solids - One of the biggest sources of water pollution in Kentucky is suspended solids.Suspended solids include inorganic particles (silts, clays, etc.) and organic particles (algae, zooplankton, bacteria, and detritus) that are carried along by water as it runs off the land. The inorganic portion is usually considerably higher than the organic. Both contribute to turbidity, or cloudiness of the water.High values of TSS cause multiple environmental impacts, including clogging fish gills, reducing light penetration, and siltation of stream bottoms and associated habitats.Indirectly, the suspended solids affect other parameters such as temperature and dissolved oxygen.Suspended solids also interfere with effective drinking water treatment. High sediment loads interfere with coagulation, filtration, and disinfection, and more chlorine is required to effectively disinfect turbid water.
There are no quantitative criteria for TSS.The Kentucky Water Quality Standards for aquatic life state that suspended solids "shall not be changed to the extent that the indigenous aquatic community is adversely affected" and "the addition of settleable solids that may adversely alter the stream bottom is prohibited."
Sulfur - Sulfur is another essential plant nutrient. Aquatic organisms utilize sulfur, and reduced concentrations have a detrimental effect on algal growth. The most common form of sulfur in well-oxygenated waters is sulfate. Sulfates (SO4--) can be naturally occurring or the result of municipal or industrial discharges. When naturally occurring, they are often the result of the breakdown of leaves that fall into a stream, of water passing through rock or soil containing gypsum and other common minerals, or of atmospheric deposition. Point sources include sewage treatment plants and industrial discharges such as tanneries, pulp mills, and textile mills. Runoff from coal mining operations and fertilized agricultural lands also contributes sulfates to water bodies.
A sulfur cycle exists, which includes atmospheric sulfur dioxide (SO2), sulfate ions (SO22-) and sulfides (S-). Sulfides, especially hydrogen sulfide (H2S), are quite soluble in water and are toxic to both humans and fish. They are produced under conditions where there is a lack of oxygen (anaerobic). Because of their foul "rotten egg" smell they are avoided by both fish and humans. Sulfides formed as a result of acid mine runoff from coal or other mineral extraction and from industrial sources may be oxidized to form sulfates, which are less toxic.
When sulfate is less than 0.5 mg/L, algal growth will not occur. On the other hand, sulfate salts can be major contaminants in natural waters.The state water quality standard for sulfate in drinking water supplies is 250 mg/L.
There is no description yet for this analyte.
There is no description yet for this analyte.
Title
Why do we care?
text
Natural occurrence
text
How much matters?
text
Human impacts on concentrations
text